Development of Population Pharmacokinetic and Adverse Event Model
Overview
This is an example of the development of a pharmacokinetic and adverse event model for a new oncology drug candidate. When testing the drug in a new indication, unexpected and unacceptable toxicity was reported. PRI developed a population pharmacokinetic model and linked the drug exposure to the likelihood of toxicity. PRI found that the drug could be administered more effectively with vitamin pretreatment, thus allowing safer use of the drug.
A Population Pharmacokinetic and Pharmacodynamic Evaluation of Pralatrexate in Patients with Hematologic MalignanciesDiane R. Mould1, Kevin Sweeney2, Stephen Duffull2,3, Ellen Neylon4, and Owen A. O’Connor41Projections Research, Phoenixville, PA 19460; 2Associates of Projections Research, Phoenixville, PA 19460; 3School of Pharmacy, University of Otago, New Zealand. 4Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, The New York Presbyterian Hospital, Columbia University, New York, N.Y
Abstract
Pralatrexate (PDX) is a novel folate analogue designed to have high affinity for the reduced folate carrier (RFC-1), exhibiting improved internalization and efficacy over other aminopterin derivatives. Early Phase 2 and subsequent Phase 1 studies revealed that patients with lymphoma exhibited a higher incidence of mucositis, compared to that reported in patients with solid tumors. The initial Phase 2 experiences employed dose escalation assessments on an every other week schedule (135 to 240 mg/m2 QOW) while the follow-on Phase 1 and 2 studies employed a weekly dosing schedule (30 to 45 mg/m2 weekly for 6 of 7 weeks). The maximum tolerated dose (MTD) was identified to be 30 mg/m2 weekly for 6 of 7 weeks in lymphoma patients. Preliminary population pharmacokinetic (PK) and adverse event (AE) evaluations suggested that PDX exposure could be controlled with weight or body size based dosing, and that pretreatment with folic acid and vitamin B12 could abrogate the mucositis. PK and AE data from 47 patients were evaluated using NONMEM Version V level 1.1. Model building followed standard approaches.
The best PK model was a three compartment linear model, fitted to the natural log-transformed concentration versus time data. Patient weight and methylmalonic acid (MMA) were found to be predictive of inter-patient PK variability, supporting weight or body surface area (BSA) based dosing. There are observations of an apparent association between MMA concentrations and PDX PK which need to be further explored.
An ordered categorical logistic regression was conducted to evaluate the relationship between PDX exposure and grade of mucositis. In this evaluation, pralatrexate area under the curve (AUC) and MMA were found to be predictive of toxicity, where patients with higher MMA or AUC had a higher probability of mucositis. Individualized dosing combined with vitamin pretreatment significantly improved the toxicity profile, with a shift in the dose-limiting toxicity (DLT) from mucositis to thrombocytopenia. Although the data from the present study were limited, the covariates identified in the population PK and AE evaluations were comparable with those identified for other anti-folate agents. MMA is an indicator of vitamin B12 deficiency and its impact on toxicity is commonly reported for this class of agents. The rationale behind the influence of MMA on clearance is less clear and may be correlated with other aspects of patient health. PDX has exhibited promising activity in patients with chemotherapy refractory peripheral T-cell lymphoma, leading to an international multi-center Phase 2 study in peripheral T-cell lymphoma. The application of population PK analysis was a critical component in the development of this agent. Model-based evaluations allowed the identification of the safest schedules of administration and supported the need for vitamin supplementation for the safe administration of this agent.
Objectives
The primary objectives of this analysis were: To characterize the PK and PK-AE relationship of PDX in the target lymphoma patient population; To identify any patient characteristics that influenced the PK of PDX and the relationship between PDX AUC and AEs; and to use the model to provide improved dose adjustment options to treat patients.
Methods
Study Design
This was an evaluation of data obtained from an open label, dose ranging Phase 1/ Phase 2 study (Study 02-078).
Patients doses could be adjusted based on tolerability.The Phase I part was to determine the MTD of PDX in lymphoma patients.
Doses of PDX ranged from 30 to 255 mg/m2. Data were available from 14 patients.
The Phase II part evaluated the effect of vitamin pretreatment.
Data were available from 33 patients. Doses ranged from 15 to 180 mg/m2 .
Pharmacokinetic and Pharmacodynamic Observations
Plasma concentrations were obtained using a dense sampling strategy during the first cycle of treatment. For remaining cycles, only trough concentrations were collected.The relationship between PDX exposure and mucositis was evaluated in the same patients. Mucositis was graded using the Common Toxicity Criteria (CTC) scale, Version 2.0. Patients were evaluated for up to 15 cycles of treatment.
Model Building Databases
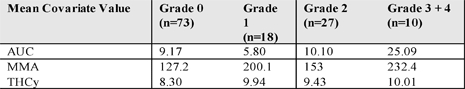
PK data from 47 lymphoma patients were used to develop the model. There were a total of 462 PDX concentrations from 47 patients included in the database. PDX concentration data were Ln-transformed for the population PK evaluation.There were 128 observations of adverse events from a total of 47 patients. There were 73 observations with no adverse event (Grade 0); 18 observations of Grade 1 events, 27 observations of Grade 2 events, 9 observations of Grade 3 events and one Grade 4 event, which was combined with the Grade 3 events The same patients were evaluated for the exposure response (PK-AE) assessment.
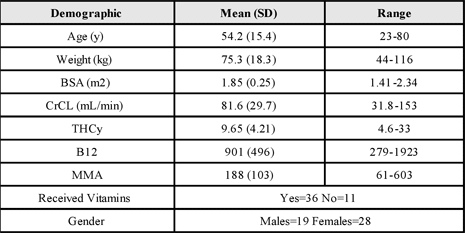
The baseline demographics for the 47 patients are listed below.
Baseline Demographics
Model Based Evaluation
Nonmem (Version V Level 1.1) compiled with Compaq Digital Visual Fortran 6.6C3 with all relevant patches was used. PK modeling used the First Order Conditional Estimation (FOCE) Method with INTERACTION. Covariates were accepted at p<0.001.
The PK-AE evaluation was performed using ordered categorical logistic regression using the Laplacian method with likelihood option.
Results
Pharmacokinetic Model
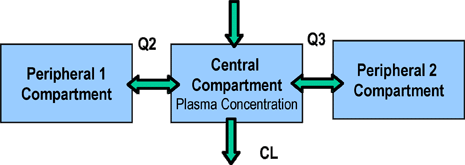
The final PK model was a three-compartment linear model. Inter-individual variance terms were estimated for all structural parameters with a full OMEGA block structure. A combination residual error model was employed. There was an effect of methylmalonic acid (MMA) on clearance, as well as an effect of body size which was included as an allometric function.
Plasma concentrations were obtained using a dense sampling strategy during the first cycle of treatment. For remaining cycles, only trough concentrations were collected.The relationship between PDX exposure and mucositis was evaluated in the same patients. Mucositis was graded using the Common Toxicity Criteria (CTC) scale, Version 2.0. Patients were evaluated for up to 15 cycles of treatment.
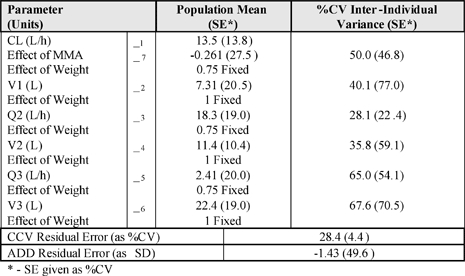
Pharmacokinetic Model
The parameter estimates for the final PK model are shown below. The standard errors were small and the bootstrapped confidence intervals (not shown) were narrow for all parameters. The estimates for Bayesian shrinkage (not shown) were all below 0.2.
Pharmacokinetic Model Diagnostics
Basic Diagnostic plots for the PK model are presented below. Model performance was generally good for all doses evaluated.

Pharmacokinetic-Adverse Event Model
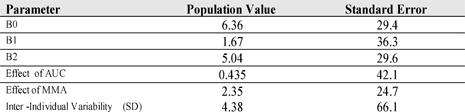
The relationship between PDX AUC and grade of mucositis was evaluated using an ordered categorical logistic model that describes the probability of an event as a function of covariates such as drug exposure and patient characteristics. The function used to describe the data was: h{P(Y≤m|h)} = fpretreat – fcovariate + ηY .Other measures of PDX exposure (actual dose, mg/m2 dose) and patient demographics were evaluated.
The logistic regression parameters are presented below. Individual patient PDX AUC and MMA were significant predictors of the probability of experiencing mucositis.
Previous data suggests that patients with high MMA were more likely to experience mucositis. Consequently, vitamin pretreatment was recommended to normalize patient MMA and improve the toxicity profile. The relationship of increasing grade of adverse event and MMA was evaluated in the present database and the results are presented below on the left.. This figure shows a shift towards less severe grade of AE with vitamin pretreatment.
The probability curves for Grade 0 and Grade 3 AE stratified by MMA for the logistic PK-AE model are shown below on the right. At a specific PDX AUC, patients with high MMA values have a substantially higher probability of experiencing mucositis than patients with normal MMA values.
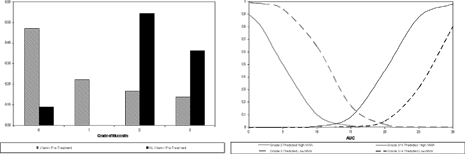
Exploration of the important predictors of toxicity by grade of AE showed that patients with higher grades of mucositis do have higher PDX AUC, and MMA further supporting the model based evaluations.
Conclusions
Patient PDX exposure is best characterized by a linear 3 compartment model.Patient weight and MMA are predictors of PK variability. The effect of weight supports dose adjustments based on body size.
PDX AUC and MMA are predictors of mucositis.
Pretreatment with vitamins (and normalizing MMA) improves toxicity.
Conflict of Interest
Drs Mould, Sweeney and Duffull were paid consultants. There are no other conflicts to disclose.